Potassium hydrogen phosphate Formula - Potassium Hydrogen Phosphate Uses, Properties, Structure and Formula
Potassium hydrogen phosphate is an inorganic chemical that belongs to the family of potassium phosphates. It is more commonly known as dipotassium phosphate or potassium phosphate dibasic.
Formula and structure: The chemical formula of potassium hydrogen phosphate is K2HPO4. Its molecular formula is HK2O4P, and its molar mass is 174.2 g/mol. It is the dipotassium salt of phosphoric acid (H3PO4, which has three acidic protons). It consists of two potassium cations (K+) and one phosphate anion (HPO42-), in which the phosphorous atom is attached to one hydroxyl group, one double bonded oxygen atom, and two single bonded oxygen atoms.
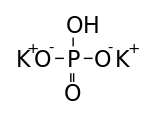
Preparation: Potassium hydrogen phosphate is prepared by the partial neutralization of phosphoric acid (H3PO4) with potassium hydroxide (KOH) or potassium carbonate (K2CO3).
2 KOH + H3PO4 → K2HPO4 + 2 H2O
Physical properties: Potassium hydrogen phosphate exists as an odorless white crystalline solid. It has a density of 2.44 g/mL and decomposes above 465 °C. It is a deliquescent solid (absorbs enough water to turn into solution).
Chemical properties: Potassium hydrogen phosphate is highly water soluble and sparingly soluble in alcohols. It is a hygroscopic and deliquescent compound, and dissolves in water to give a moderately basic solution. It is a stable compound but decomposes when heated to high temperatures.
Uses: Potassium hydrogen phosphate is mainly used as a fertilizer, corrosion inhibitor and buffering agent. It is also used as a food additive, in mineral supplements, in non-dairy creamers, and in pharmaceuticals. It is used as an electrolyte source and has radioprotective activity. Other applications include paper processing, yeast nutrient, agar for culturing bacteria and chemical reagent.
Health effects/safety hazards: Potassium hydrogen phosphate can irritate the skin, eyes and respiratory tract upon contact or inhalation. It is not toxic and is considered safe as a food additive. However, excessive consumption of potassium salts can cause imbalance in the electrolyte levels, resulting in several symptoms such as nausea, vomiting, diarrhea, abdominal discomfort, and limited absorption of necessary nutrients.
|
Related Links: |
Related Topics
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
Yeast Facts
Calcium Facts
Ionic and Net Ionic Equations
